Medical Professionals
This webpage contains scientific information meant for medical professionals only.
Improving cognition and daily functioning in Vascular Dementia

Indication
CognivAiD is indicated for patients with mild to moderate vascular dementia.
Composition
CognivAiD is a multi-herbal formulation containing active extracts of 3 ingredients:
• Croci Stigma
• Ginkgo Folium
• Ginseng Radix et Rhizoma
Dosage & administration
The recommended dosage is 2 capsules, 2 times a day orally.
Side effects
Rare cases of headaches, gastrointestinal disturbances or allergic skin reactions.
Precautions
Not recommended in pregnant and lactating women, and in individuals with bleeding tendency or are hypersensitive to ginkgo. Not recommended for concomitant use with anticoagulants/antiplatelets, and before surgery.
A targeted multi-modal Action
CognivAiD simultaneously targets the key pathological pathways of vascular dementia.

References:
1. Seto, S., et al. (2017). Sailuotong prevents hydrogen peroxide (H2O2)-induced injury in EA.hy926 cells. International Journal of Molecular Sciences, 18(1), 95. https://doi.org/10.3390/ijms18010095
2. Yeon, S. Y., et al. (2020). Endothelium-Independent Vasodilatory Effect of Sailuotong (SLT) on Rat Isolated Tail Artery. Evidence-Based Complementary and Alternative Medicine, 2020, 1–10. https://doi.org/10.1155/2020/8125805
3. Zhang, Y., et al. (2019). Sailuotong Capsule Prevents the Cerebral Ischaemia-Induced Neuroinflammation and Impairment of Recognition Memory through Inhibition of LCN2 Expression. Oxidative Medicine and Cellular Longevity, 2019, 1–13. https://doi.org/10.1155/2019/8416105
Sustained Improvement of Functional & Cognitive Domains in
Vascular Dementia (VaD) With a Favourable Safety Profile
Beneficial as Monotherapy
1. Improves cognitive and executive functions1
• Significant improvement of cognitive function based on VaDAS-Cog assessment as early as 3 months
• Long-term continuous improvement up to 1 year
• Reproducible benefits seen in delayed-starter groups after switching from placebo to CognivAiD
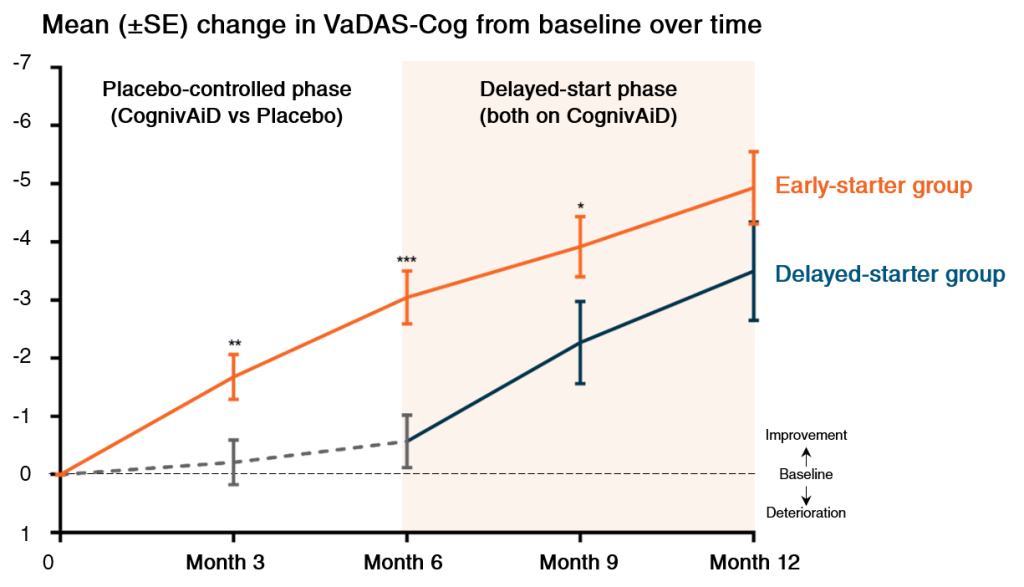
VaDAS-Cog: Vascular Dementia Assessment Scale Cognitive subscale
2. Significant & clinically meaningful global improvement1
• Significant improvement in global condition of patients assessed by clinician with input of caregiver, based on ADCS-CGIC
• Divergence of outcomes between early- and delayed-starter groups was reversed as early as 3 months after switching from placebo to CognivAiD in delayed-starter groups
• Sustained improvement for at least 1 year
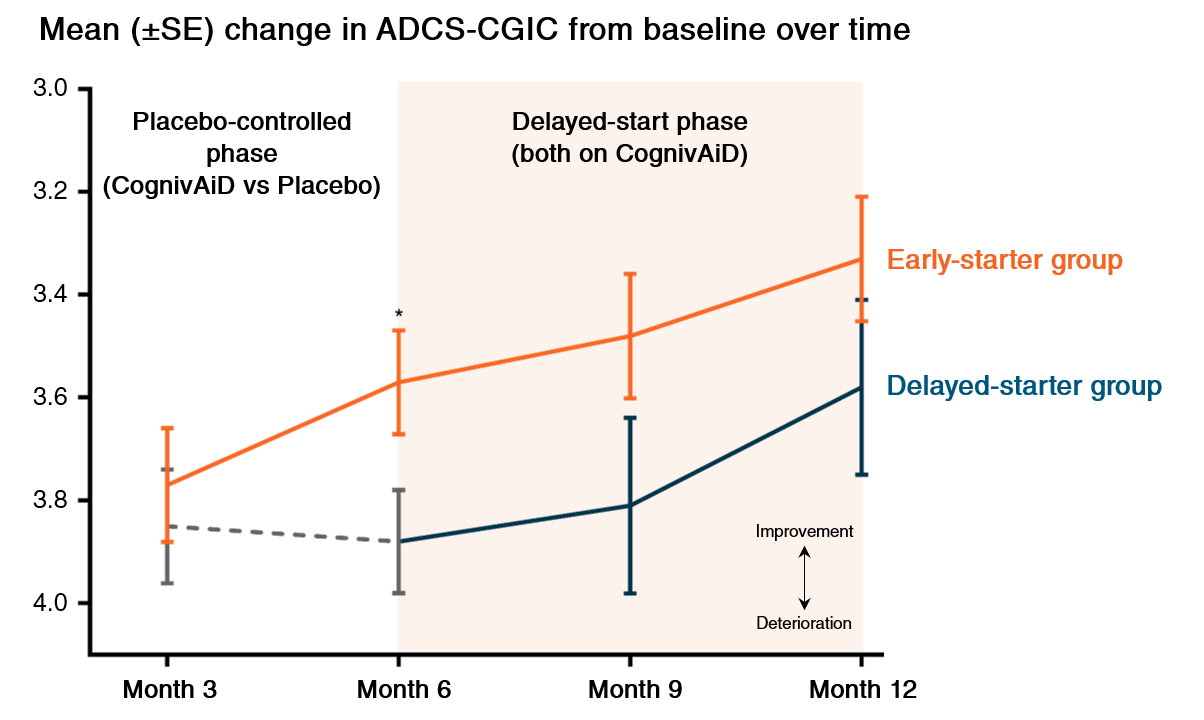
ADCS-CGIC: Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change
3. Delays functional decline1
• CognivAiD reduces the proportion of deteriorating patients by 47% vs placebo.
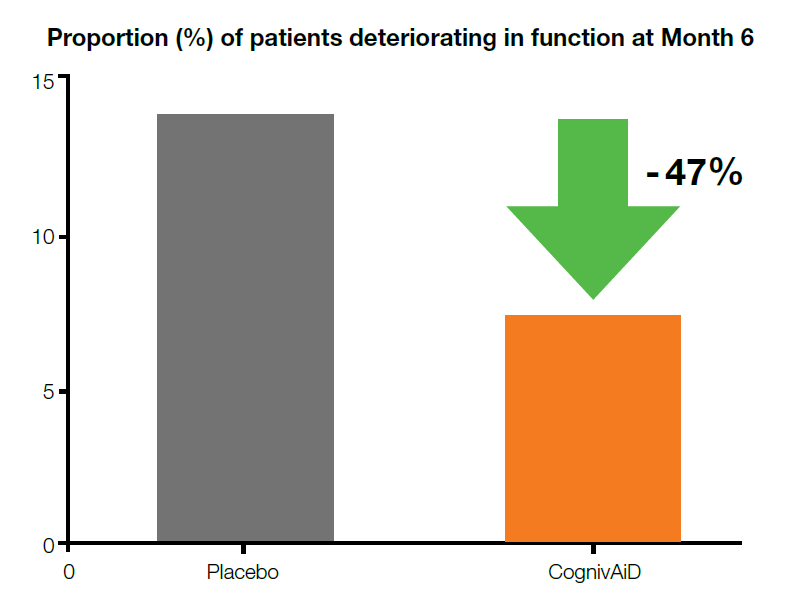
Legend:
*p<0.05, **p<0.01, ***p<0.001
References
1. Jia, J., et al. (2018). Efficacy and safety of the compound Chinese medicine SaiLuoTong in vascular dementia: A randomized clinical trial. Alzheimer’s & dementia (New York, N. Y.), 4, 108–117. https://doi.org/10.1016/j.trci.2018.02.004
Beneficial as Add-on
1. Enhances cognition2
• Significant & clinically meaningful improvement in cognition compared to placebo
• Sustained improvements while receiving CognivAiD
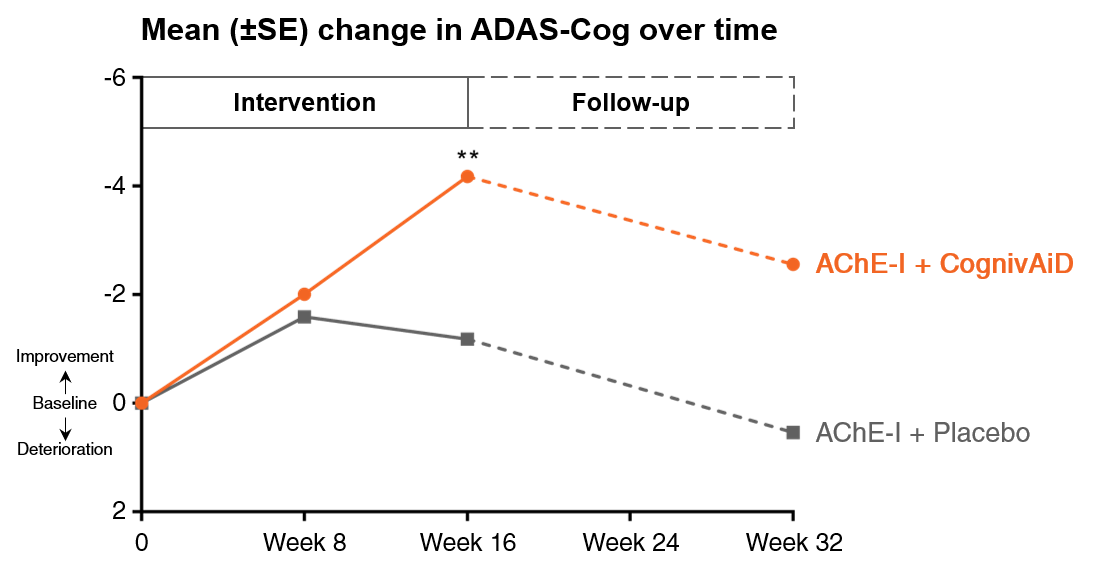
ADAS-Cog: Alzheimer’s Disease Assessment Scale Cognitive subscale
2. Enhances daily functioning2
• CognivAiD nearly doubles the proportion of subjects who improved functionally vs placebo.
• CognivAiD reduces by 38% the proportion of deteriorating subjects vs placebo.
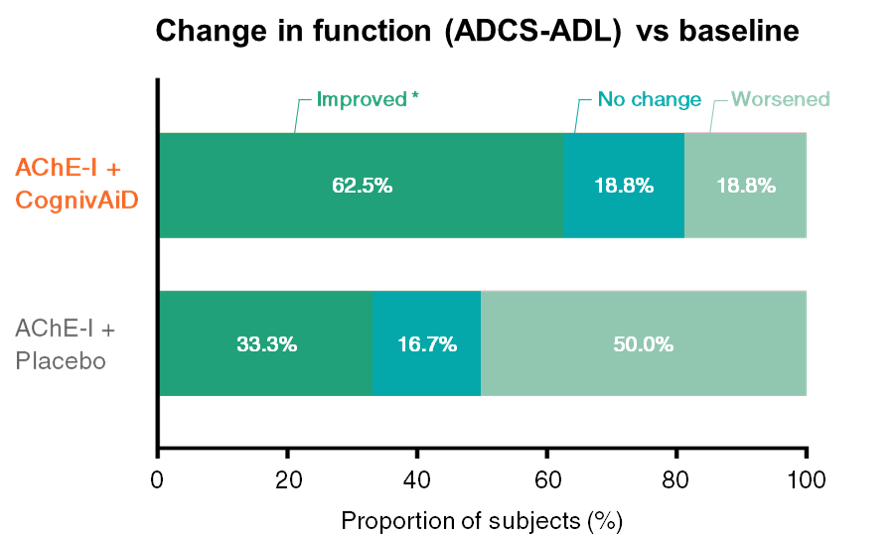
ADCS-CGIC: Alzheimer's Disease Cooperative Study-Activities of Daily Living
3. Improves quality of life2
• CognivAiD nearly triples the proportion of subjects who reported better quality of life vs placebo.
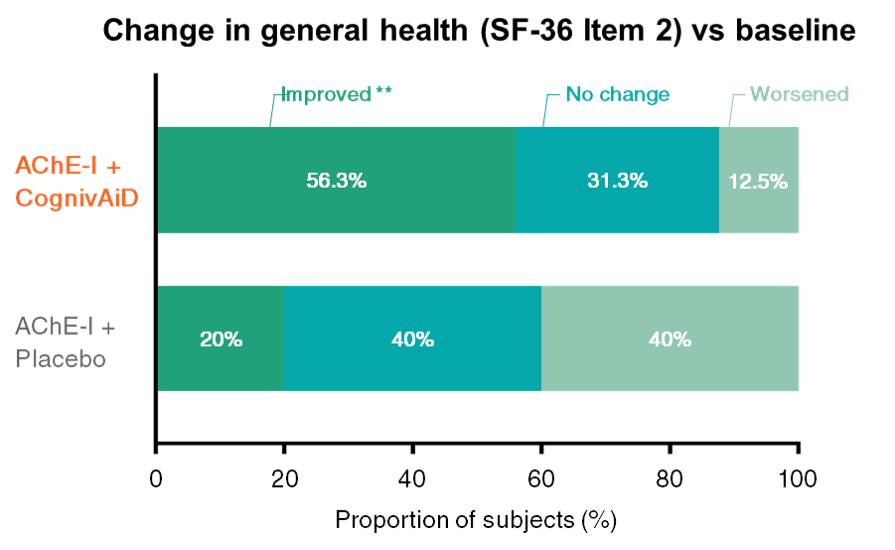
SF-36: Short Form 36 Health Survey Questionnaire
Legend:
*p<0.05, **p<0.01
References
2. Liu, J., et al. (2022). A pilot randomized controlled trial of WeiNaoKang (SaiLuoTong) in treating vascular dementia. Aging medicine (Milton (N.S.W)), 5(4), 246–256. https://doi.org/10.1002/agm2.12230
Safe & Well-Tolerated
1. Safe and well-tolerated
• No significant difference in rate of adverse events in those taking CognivAiD vs placebo.1,2
References
1. Jia, J., et al. (2018). Efficacy and safety of the compound Chinese medicine SaiLuoTong in vascular dementia: A randomized clinical trial. Alzheimer’s & dementia (New York, N. Y.), 4, 108–117. https://doi.org/10.1016/j.trci.2018.02.004
2. Liu, J., et al. (2022). A pilot randomized controlled trial of WeiNaoKang (SaiLuoTong) in treating vascular dementia. Aging medicine (Milton (N.S.W)), 5(4), 246–256. https://doi.org/10.1002/agm2.12230
Get in touch with us
For more information, you may email your enquiries directly to [email protected]. Alternatively, you may fill in our enquiry form.

